Antibody-Drug Conjugates
|
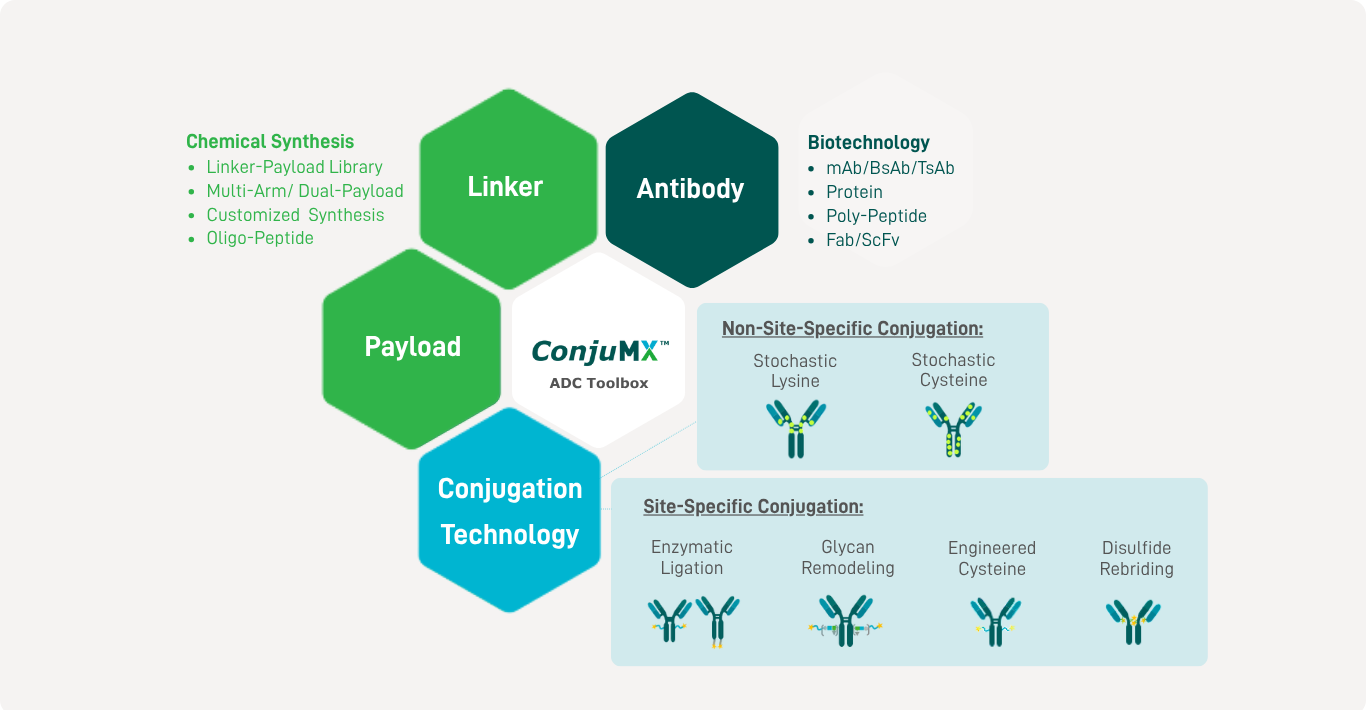
Mycenax collaborates to provide a comprehensive, one-stop Antibody-Drug Conjugate (ADC) CDMO services, covering the development and production of antibodies, linkers-payloads, and ADCs. These integrated services span from candidate ADC selection and process development to GMP manufacturing. Leveraging the ConjuMX™ Toolbox, we offer a variety of conjugation methods and optimized processes to accelerate and shorten the timeline for new drug development. This platform is also well-suited for the development needs of novel conjugated drugs. |
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
One-stop CDMO services to accelerate drug development |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Mycenax offers end-to-end solutions spanning from antibody cell line development, linker/drug chemical synthesis to conjugation processes. We provide flexible, stage-specific services tailored to client needs, integrating small-scale experiments through commercial production. This significantly reduces coordination costs while ensuring stable supply of antibodies (Ab) and linker-payloads. Our optimized, validated processes enable seamless GMP-scale manufacturing readiness. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
ConjuMX™ Toolbox: Diverse Expertise for Customized Solutions |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The ConjuMX™ Toolbox enables rapid screening of diverse linker-payload combinations and conjugation technologies during early development. It facilitates rapid generation of ADC samples for feasibility studies and analysis. Leveraging our ConjuMX™ Toolbox, we bring expertise from over 25 ADCs, achieving GMP-grade 50L scale production. Our innovative pipeline includes advancements in bispecific ADCs (BsADCs), trispecific ADCs (TsADCs), and dual-payload ADCs. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Next-generation ADCs: Leveraging Proprietary Multi-Arm Linker Technology to Develop Multi-Arm ADCs or Dual-Payload ADCs. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Mycenax has successfully applied Mycenax’s Multi-Arm and Dual-Payload Linkers in ADC development, bringing new possibilities and added value for next-generation ADCs. The Multi-Arm Linker is designed to enhance drug loading capacity, while the Dual-Payload Linker represents a critical innovation for addressing ADC drug resistance. By harnessing the synergistic or additive effects of dual payloads, this technology can overcome tumor heterogeneity and drug resistance, paving the way for more effective cancer therapies. ▶️ Video Introduction: https://youtu.be/thcFkiu4U6A |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
One Strategic Partnerships |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
KriSan offers small molecule drugs, linkers, payloads, and GMP production for ADC drugs for Mycenax’s global Antibody-Drug Conjugates CDMO business. Mycenax and KriSan announce a collaboration to strengthen their one-stop service chain for ADC. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Mycenax collaborates to provide a comprehensive, one-stop Antibody-Drug Conjugate (ADC) CDMO services, covering the development and production of antibodies, linkers-payloads, and ADCs. These integrated services span from candidate ADC selection and process development to GMP manufacturing. Leveraging the ConjuMX™ Toolbox, we offer a variety of conjugation methods and optimized processes to accelerate and shorten the timeline for new drug development. This platform is also well-suited for the development needs of novel conjugated drugs.  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
One-stop CDMO services to accelerate drug development |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Mycenax offers end-to-end solutions spanning from antibody cell line development, linker/drug chemical synthesis to conjugation processes. We provide flexible, stage-specific services tailored to client needs, integrating small-scale experiments through commercial production. This significantly reduces coordination costs while ensuring stable supply of antibodies (Ab) and linker-payloads. Our optimized, validated processes enable seamless GMP-scale manufacturing readiness. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
ConjuMX™ Toolbox: Diverse Expertise for Customized Solutions |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The ConjuMX™ Toolbox enables rapid screening of diverse linker-payload combinations and conjugation technologies during early development. It facilitates rapid generation of ADC samples for feasibility studies and analysis. Leveraging our ConjuMX™ Toolbox, we bring expertise from over 25 ADCs, achieving GMP-grade 50L scale production. Our innovative pipeline includes advancements in bispecific ADCs (BsADCs), trispecific ADCs (TsADCs), and dual-payload ADCs. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Next-generation ADCs: Leveraging Proprietary Multi-Arm Linker Technology to Develop Multi-Arm ADCs or Dual-Payload ADCs. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Mycenax has successfully applied Mycenax’s Multi-Arm and Dual-Payload Linkers in ADC development, bringing new possibilities and added value for next-generation ADCs. The Multi-Arm Linker is designed to enhance drug loading capacity, while the Dual-Payload Linker represents a critical innovation for addressing ADC drug resistance. By harnessing the synergistic or additive effects of dual payloads, this technology can overcome tumor heterogeneity and drug resistance, paving the way for more effective cancer therapies. ▶️ Video Introduction: https://youtu.be/thcFkiu4U6A |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
One Strategic Partnerships |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

KriSan offers small molecule drugs, linkers, payloads, and GMP production for ADC drugs for Mycenax’s global Antibody-Drug Conjugates CDMO business. Mycenax and KriSan announce a collaboration to strengthen their one-stop service chain for ADC. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||





