Formulation Development
|
Mycenax delivers rapid and comprehensive drug product development, specializing in liquid and lyophilized formulations for diverse biotherapeutics. Offering end-to-end solutions, from drug substance to drug product, we ensure maximized filling success with expert drug process development and a wide range of dosage form expertise, including vials and prefilled syringes.
Highlights of formulation development experience:
|
||||||||||
|
||||||||||
 |
||||||||||
|
Comprehensive Drug Product Development Expertise |
||||||||||
|
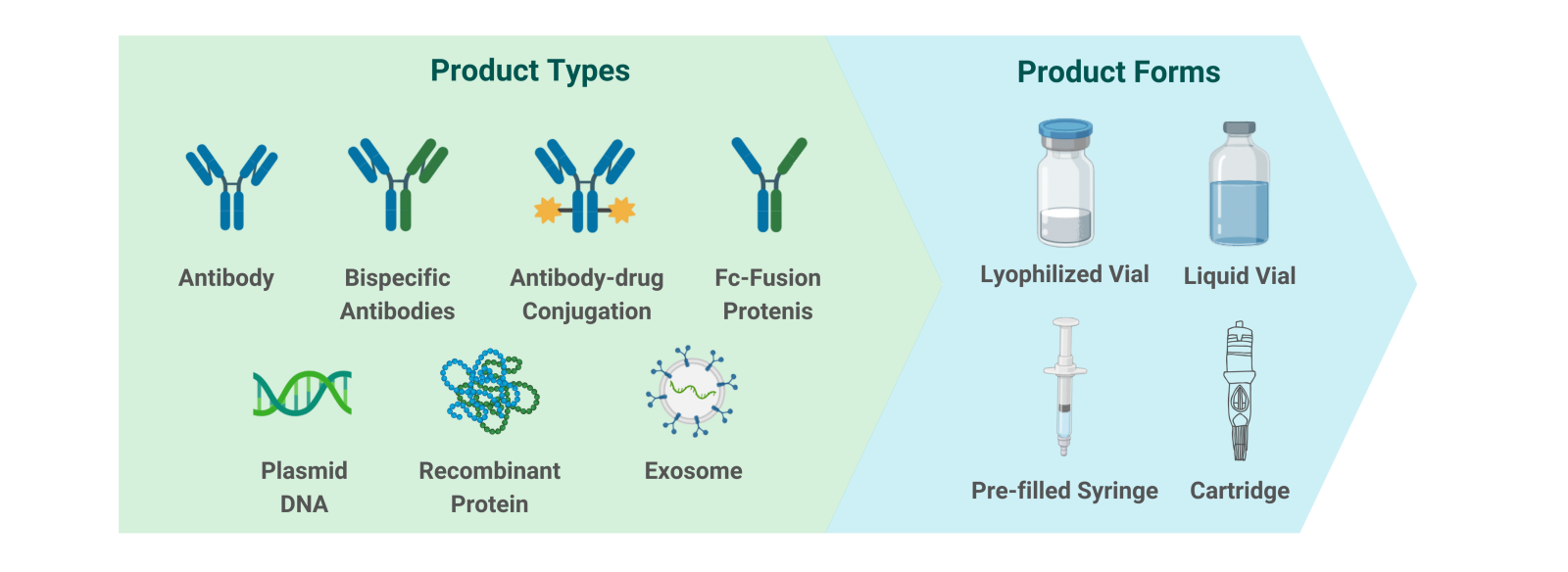
We provide comprehensive dosage form design and development services, leveraging our extensive experience across all phases of clinical development. Our capabilities span a wide range of therapeutic modalities, including antibodies, bispecific antibodies, and emerging therapies like exosomes and recombinant proteins, with experience in lyophilized formulations and various container formats, including vials and pre-filled syringes. |
||||||||||
 |
||||||||||
 |
||||||||||
|
End-to-End Drug Product Development Strategies |
||||||||||
|
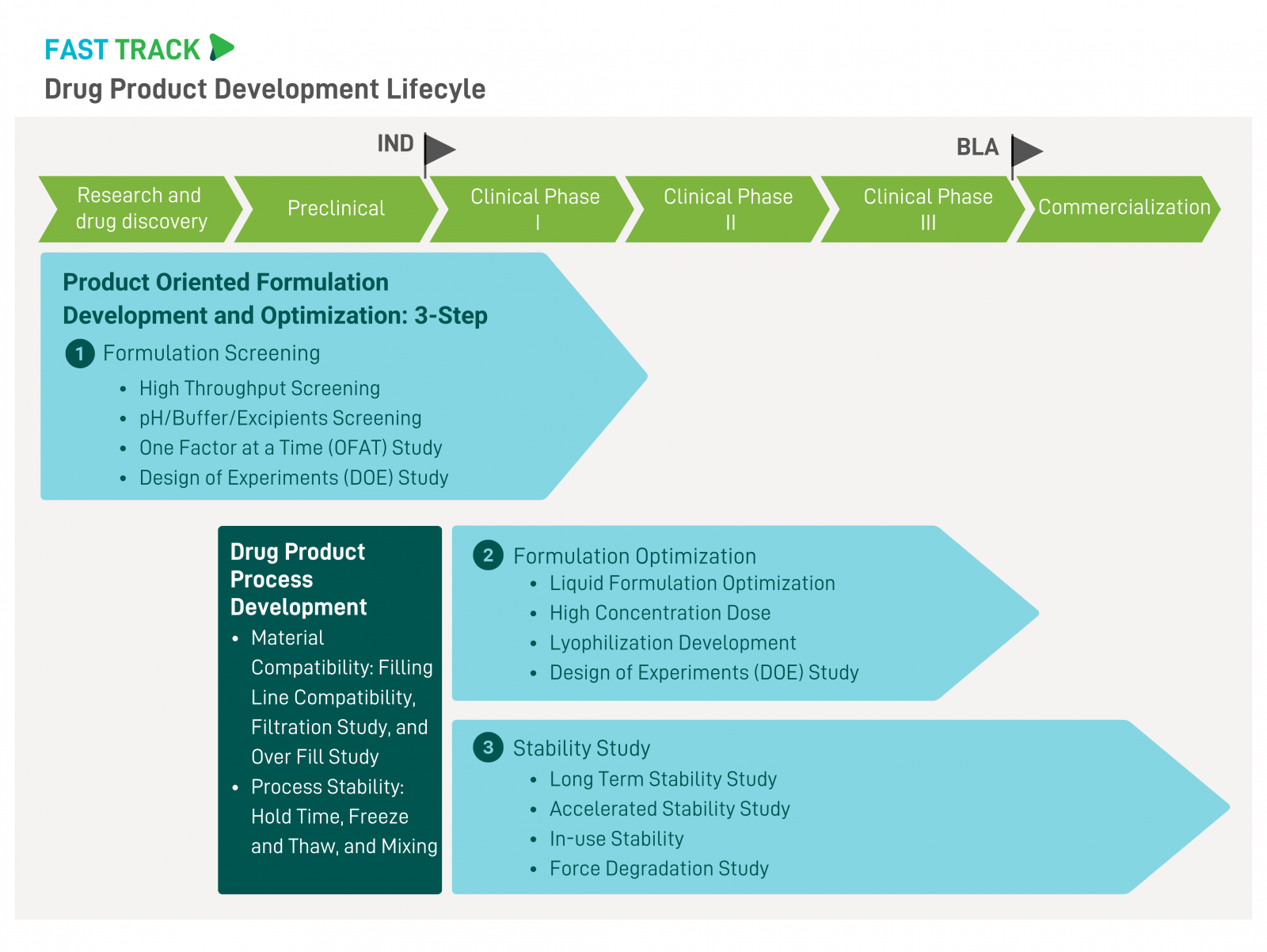
Mycenax offers formulation development services tailored to various biotherapeutic product types, addressing the needs at every stage of the product lifecycle, including pre-IND, clinical stages, and post-market phases. In the pre-IND phase, we provide formulation development by making informed decisions based on a deep understanding of the product's properties and characteristics. Both Design of Experiments (DOE) and One Factor at a Time (OFAT) approaches are utilized based on the design. Liquid formulations and lyophilized forms can be developed at the lab scale and scaled up to manufacturing levels. |
||||||||||
|
Our expert team has experience in formulation development across various product types. Based on the drug development lifecycle, Mycenax offers FAST TRACK solutions with the following services:
|
||||||||||
 |
||||||||||
|
Maximizing Filling Success: Drug Process Development Services |
||||||||||
|
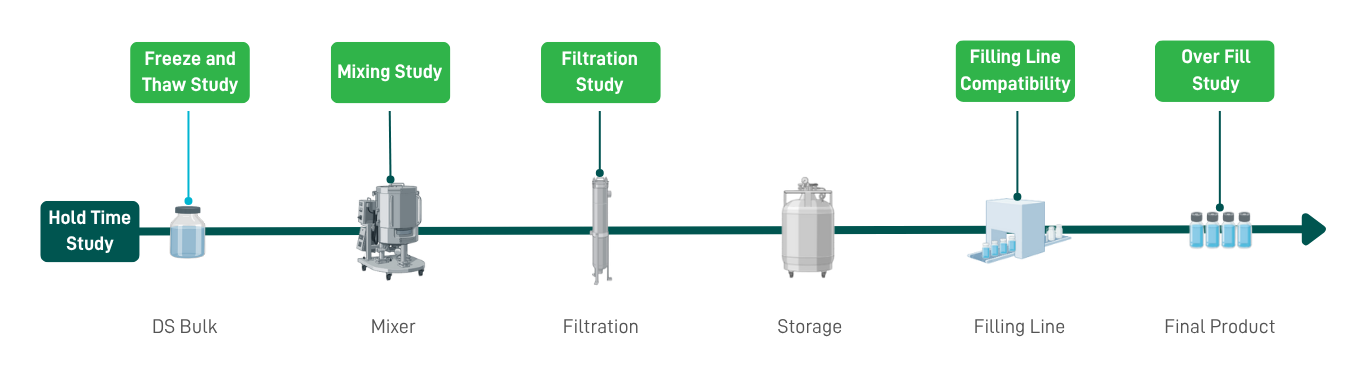
To maximize a successful and expedited filling process, drug process development must be carried out prior to product filling. Our expert team offers the following services to confirm the product's stability during the filling process and its compatibility with the filling line materials. |
||||||||||
 |
||||||||||
 |
||||||||||

